Trending...
- Unauthorized Charge Dispute Raises Abuse Concerns in North Riverside Loan Office
- Lick Personal Oils Introduces the Ultimate Valentine's Day Gift Collection for Romantic, Thoughtful Gifting
- Indies United is pleased to present our January 2026 book releases
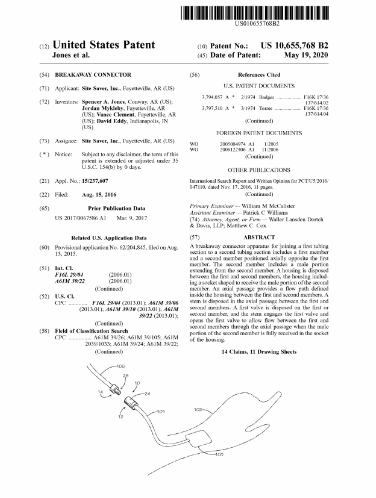
FAYETTEVILLE, Ark. - illiNews -- Lineus Medical continues to expand the patent protection of its flagship product, SafeBreak Vascular, a break-away medical device technology that helps protect IV lines. The company was awarded United States utility patent US10655768B2 that covers several key features of the device. This is the third patent awarded to Lineus Medical, which now has two utility patents and one design patent pertaining to SafeBreak Vascular.
SafeBreak Vascular is a first of its kind breakaway medical device designed for peripheral IV lines. When a damaging level of force is applied to an IV line, SafeBreak Vascular purposefully separates to minimize the effects of the damaging force and to prevent patient bleeding and the spillage of medication. Vance Clement, CEO of Lineus Medical said, "This issuance of this new utility patent is another important step in adding value to our company and protecting our first product to market, SafeBreak Vascular. The portfolio of patents the company has secured puts the company in a strong intellectual property position to protect and defend its value and SafeBreak Vascular from competition."
More on illi News
Spencer Jones, Chief Technology Officer and Founder of Lineus Medical commented, "We continue to build an intellectual property moat around SafeBreak Vascular, and that moat just became more difficult to overcome. Innovation in product design must be accompanied by meaningful patent protection, and we expect that our issued patents and patents currently under review will further protect our technologies and set-us apart for many years in the market place."
ABOUT LINEUS MEDICAL
Lineus Medical is a developer of medical device technologies that serve the vascular access/IV market. The company develops products that help prevent pain and discomfort in patients, save nurses time and control costs for hospitals. SafeBreak Vascular is not yet FDA cleared and is not yet for sale. More information about Lineus Medical is available at www.lineusmed.com.
MKG-0068 Rev 00
SafeBreak Vascular is a first of its kind breakaway medical device designed for peripheral IV lines. When a damaging level of force is applied to an IV line, SafeBreak Vascular purposefully separates to minimize the effects of the damaging force and to prevent patient bleeding and the spillage of medication. Vance Clement, CEO of Lineus Medical said, "This issuance of this new utility patent is another important step in adding value to our company and protecting our first product to market, SafeBreak Vascular. The portfolio of patents the company has secured puts the company in a strong intellectual property position to protect and defend its value and SafeBreak Vascular from competition."
More on illi News
- Litera's Agentic AI Drives 10x User Growth in 3 Months since Launching
- Breakout Phase for Public Company: New Partnerships, Zero Debt, and $20 Million Growth Capital Position Company for 2026 Acceleration
- Japan's Patented "Hammock'n" Smartphone Band Targets Hand Fatigue From Long Phone Use
- Reditus Group Introduces A New Empirical Model for Early-Stage B2B Growth
- CCHR: Harvard Review Exposes Institutional Corruption in Global Mental Health
Spencer Jones, Chief Technology Officer and Founder of Lineus Medical commented, "We continue to build an intellectual property moat around SafeBreak Vascular, and that moat just became more difficult to overcome. Innovation in product design must be accompanied by meaningful patent protection, and we expect that our issued patents and patents currently under review will further protect our technologies and set-us apart for many years in the market place."
ABOUT LINEUS MEDICAL
Lineus Medical is a developer of medical device technologies that serve the vascular access/IV market. The company develops products that help prevent pain and discomfort in patients, save nurses time and control costs for hospitals. SafeBreak Vascular is not yet FDA cleared and is not yet for sale. More information about Lineus Medical is available at www.lineusmed.com.
MKG-0068 Rev 00
Source: Lineus Medical
0 Comments
Latest on illi News
- WGN Chicago Features Shantel Love's Promote Your D@mn Self™ in Elevate Box Spotlight Segment
- American Laser Study Club Announces 2026 Kumar Patel Prize in Laser Surgery Recipients: Ann Bynum, DDS, and Boaz Man, DVM
- Lineus Medical Completes UK Registration for SafeBreak® Vascular
- Canyons & Chefs Announces Revamped Homepage
- $140 to $145 Million in 2026 Projected and Profiled in New BD Deep Research Report on its Position in $57 Billion US Marine Industry; N Y S E: OTH
- Glitters and Grace Expands Event Experiences Across Chicago
- Really Cool Music Releases Its Fourth Single - "So Many Lost Years"
- MGN Logistics Acquires Fast Service LLC, Fueling MyMGN Marketplace Expansion and Supercharging Expedited Coverage Nationwide
- The Wait is Over: Salida Wine Festival Announces Triumphant 2026 Return After Seven-Year Hiatus
- Graduates With $40K in Student Debt Are Buying Businesses Instead of Taking Entry-Level Jobs
- Anne Seidman: Within the Lines
- How Democrats Made Healthcare More Expensive in 2026
- Inkdnylon Launches Bilingual Ask Inkdnylon Platform
- JS Gallery Brings Global Voices to LA Art Show 2026 with "OFF SCRIPT" Exhibition
- Chicago Man, 45, is Allegedly Caught on Camera Breaking Into a West Pullman Home on New Year's Day
- ANTOANETTA Partners With Zestacor Digital Marketing to Expand Online Presence for Handcrafted Luxury Jewelry
- Digi 995 Launches as an Independent Digital Universe Powered by Community and Creator Vision
- Litchfield Cavo Announces Six Partners Elected for 2026
- FrostSkin Launches Kickstarter Campaign for Patent-Pending Instant-Chill Water Purification Bottle
- The New Monaco of the South (of Italy)