Trending...
- Chicago: Mayor Brandon Johnson and CDPH Commissioner Ige Statement On Trump Cutting Billions In Mental Health And Addiction Services Funding
- Chicago: Mayor Brandon Johnson Statement On Trump Revoking Protected Status For Somali Immigrants
- Naperville Police Arrest Two Teens Charged with Sexually Assaulting a Female
$NRXP Has Manufactured Multiple Commercial Lots of NRX-100 and KETAFREE™ with a Shelf Life of Three Years.
MIAMI - illiNews -- NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is rapidly emerging as one of the most compelling growth stories in mental-health therapeutics. With three revenue-generating treatment facilities now operating in Florida—and six expected by year-end—the company is entering 2026 with accelerating clinical operations, expanding market share, and advancing two FDA-directed regulatory pathways for ketamine-based therapeutics aimed at suicidal depression, one of the largest unmet needs in mental health.
Backed by strong clinical data, FDA Fast Track designations, and third-party revenue projections, NRXP appears positioned to capitalize on a rapidly expanding U.S. ketamine market currently estimated at $750 million, projected to reach $3.35 billion globally by 2034.
Adding to investor enthusiasm, analyst D. Boral issued a Buy rating and a $34 price target, citing NRXP's accelerating execution and diversified commercialization strategy.
A Mission Targeting One of America's Most Pressing Health Crises
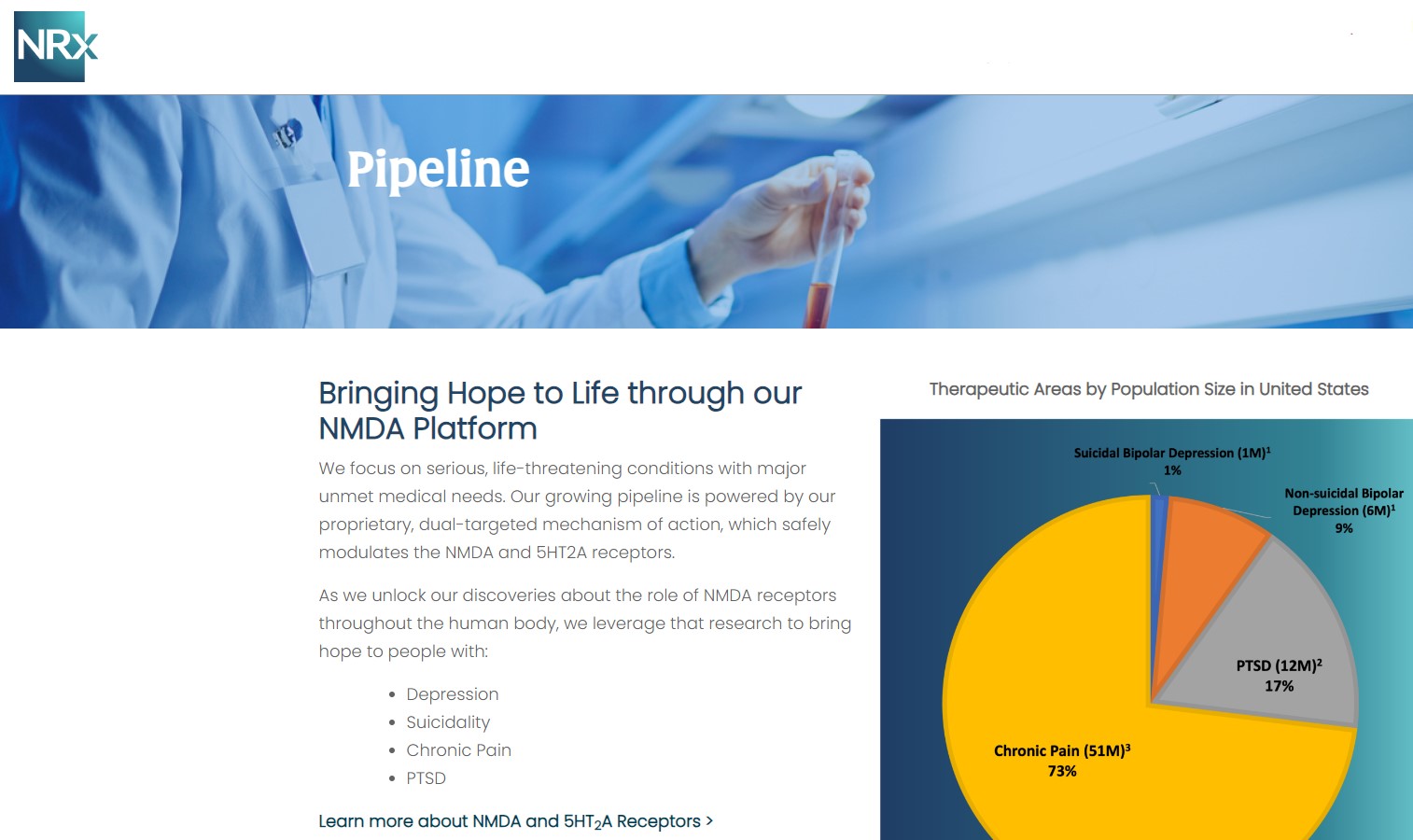
More than 13 million Americans seriously consider suicide each year (CDC). NRXP is developing next-generation therapeutics aimed directly at this crisis through its NMDA-based drug platform:
Key Pipeline Assets
NRXP is partnered with Alvogen Pharmaceuticals for the development and commercialization of NRX-101.
More on illi News
A Dual-Path Strategy for NRX-100: Innovative and Generic Market Entry
NRXP's regulatory strategy is unique: it is simultaneously pursuing both an innovative NDA pathway and a generic ANDA pathway for NRX-100 and KETAFREE™.
This creates two powerful value drivers:
1. Innovative NDA for Suicidal Depression
2. ANDA for KETAFREE™ (Generic Ketamine)
With commercial lots already manufactured, NRXP is positioned for a swift commercial rollout upon approval.
HOPE Clinics: Expanding Revenue Footprint Across Florida
NRXP's HOPE Therapeutics subsidiary is advancing a scalable clinic model for ketamine and advanced TMS-based interventions.
Current & Expected Expansion
These facilities support:
Management expects increased revenue contributions from clinical operations through 2026.
Breakthrough ONE-D: First-in-Florida Deployment with Ampa Health
In November, NRXP launched patient treatment using Ampa Health's ONE-D protocol—a groundbreaking approach reporting:
ONE-D combines:
ONE-D is being deployed at HOPE clinics in Sarasota, Naples, and Fort Myers, with additional locations coming online in 2025.
More on illi News
This marks a major competitive differentiator for NRXP in the mental-health treatment sector.
Financial Position: Capital Secured Through July 2026
NRXP reported securing sufficient operating capital to support its drug development programs through July 2026, complementing increasing clinical revenues.
Third-quarter results (released November 17, 2025) highlighted:
The full Q3 update and webcast are available on the NRXP investor relations site.
High-Value Licensing Opportunity
NRXP has accepted non-binding potential terms from an international pharmaceutical partner for the licensing and distribution of NRX-100. Terms include:
If finalized, this could represent a transformative revenue opportunity for the company.
Investment Outlook
With:
NRx Pharmaceuticals is attracting increased attention from both institutional and retail investors.
The recent $34 price target from D. Boral underscores growing confidence in NRXP's ability to execute on a scalable, high-margin strategy targeting one of the most underserved and urgent areas of mental health.
For More Information
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Website: www.nrxpharma.com
Chief Business Officer: Matthew Duffy
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Backed by strong clinical data, FDA Fast Track designations, and third-party revenue projections, NRXP appears positioned to capitalize on a rapidly expanding U.S. ketamine market currently estimated at $750 million, projected to reach $3.35 billion globally by 2034.
Adding to investor enthusiasm, analyst D. Boral issued a Buy rating and a $34 price target, citing NRXP's accelerating execution and diversified commercialization strategy.
A Mission Targeting One of America's Most Pressing Health Crises
More than 13 million Americans seriously consider suicide each year (CDC). NRXP is developing next-generation therapeutics aimed directly at this crisis through its NMDA-based drug platform:
Key Pipeline Assets
- NRX-100 (IV Ketamine):
- FDA Fast Track designation for suicidal ideation in depression, including bipolar depression.
- Multiple commercial lots produced with three-year shelf life stability.
- NDA submission expected in Q4 2025, supported by real-world patient data from more than 60,000 IV ketamine cases.
- KETAFREE™ (preservative-free ketamine):
- Pursuing approval via an ANDA (generic) pathway.
- Recently received supportive FDA correspondence confirming no major deficiencies and on track for a Q2 2026 GDUFA date.
- Addresses a ketamine market worth ~$750 million annually.
- NRX-101 (D-cycloserine + lurasidone):
- FDA-designated Investigational Breakthrough Therapy.
- Targets suicidal treatment-resistant bipolar depression, chronic pain, and potential utility in complicated UTIs.
- New real-world data suggest its active ingredient doubles the effectiveness of TMS—a potential major indication expansion.
NRXP is partnered with Alvogen Pharmaceuticals for the development and commercialization of NRX-101.
More on illi News
- Still Searching for the Perfect Valentine's Gift? Lick Personal Oils Offers Romantic, Experience-Driven Alternatives to Traditional Presents
- Boston Industrial Solutions' BPA Certified BX Series Raises the Bar for Pad Printing Inks
- Nationwide Dog-Friendly Restaurant Directory Launches with 5,000+ Listings
- Boston Corporate Coach™ Sets Global Standard for Executive Chauffeur Services Across 680 Cities
- UK Financial Ltd Announces CoinMarketCap Supply Verification And Market Positioning Review For Regulated Security Tokens SMPRA And SMCAT
A Dual-Path Strategy for NRX-100: Innovative and Generic Market Entry
NRXP's regulatory strategy is unique: it is simultaneously pursuing both an innovative NDA pathway and a generic ANDA pathway for NRX-100 and KETAFREE™.
This creates two powerful value drivers:
1. Innovative NDA for Suicidal Depression
- Expected completion: Q4 2025.
- Includes comparative real-world data showing IV ketamine may have faster onset and greater effect than intranasal S-ketamine.
- NRXP has applied for a Commissioner's National Priority Voucher, which could accelerate FDA review.
2. ANDA for KETAFREE™ (Generic Ketamine)
- Re-filed after FDA approved NRXP's Suitability Petition for its preservative-free formulation.
- Supportive FDA feedback in November 2025 indicated no significant deficiencies.
- A Citizen Petition filed to remove toxic preservative benzethonium chloride from ketamine products could reshape the competitive landscape.
With commercial lots already manufactured, NRXP is positioned for a swift commercial rollout upon approval.
HOPE Clinics: Expanding Revenue Footprint Across Florida
NRXP's HOPE Therapeutics subsidiary is advancing a scalable clinic model for ketamine and advanced TMS-based interventions.
Current & Expected Expansion
- 3 operational facilities in Florida
- 6 additional facilities planned by year-end
These facilities support:
- NRX-100 Expanded Access Program
- Ketamine-based clinical services
- ONE-D single-day TMS depression treatments
- Veterans, first responders, and active-duty military populations
Management expects increased revenue contributions from clinical operations through 2026.
Breakthrough ONE-D: First-in-Florida Deployment with Ampa Health
In November, NRXP launched patient treatment using Ampa Health's ONE-D protocol—a groundbreaking approach reporting:
- 87% response rate
- 72% remission
- Achieved in one day, instead of traditional 90-day TMS schedules
ONE-D combines:
- Ampa's FDA-cleared TMS device
- D-cycloserine (NRX-101 active ingredient)
- Lisdexamfetamine
ONE-D is being deployed at HOPE clinics in Sarasota, Naples, and Fort Myers, with additional locations coming online in 2025.
More on illi News
- Naperville Police Accepting Applications for Spring Citizen Police Academy
- Sharpe Automotive Redefines Local Car Care with "Transparency-First" Service Model in Santee
- Chicago: ICYMI: Mayor Brandon Johnson Builds On Community Safety, Violence, And Overdose Reduction Progress—Blasts Trump Cuts
- Indie Creator Opens the Digi 995 Universe Ahead of Kickstarter Launch
- Chicago: Mayor Brandon Johnson Announces New Record Of Nearly 13 Million Shared Bike And Scooter Trips In 2025
This marks a major competitive differentiator for NRXP in the mental-health treatment sector.
Financial Position: Capital Secured Through July 2026
NRXP reported securing sufficient operating capital to support its drug development programs through July 2026, complementing increasing clinical revenues.
Third-quarter results (released November 17, 2025) highlighted:
- Progress across all clinical and regulatory objectives
- Expanded Fast Track designation for NRX-100
- EU and NIH data enhancing regulatory strategy
- Growth of the HOPE facility platform
- Enhanced IP and formulation positioning
The full Q3 update and webcast are available on the NRXP investor relations site.
High-Value Licensing Opportunity
NRXP has accepted non-binding potential terms from an international pharmaceutical partner for the licensing and distribution of NRX-100. Terms include:
- Over $300 million in milestone payments
- Tiered double-digit royalties
If finalized, this could represent a transformative revenue opportunity for the company.
Investment Outlook
With:
- Multiple clinical catalysts approaching
- Two regulatory pathways advancing for NRX-100
- FDA Breakthrough Therapy designations
- National leadership in cutting-edge TMS + D-cycloserine therapy
- Increasing real-world data support
- A growing network of revenue-producing treatment centers
- Newly manufactured commercial drug supply
- And a strong capital runway
NRx Pharmaceuticals is attracting increased attention from both institutional and retail investors.
The recent $34 price target from D. Boral underscores growing confidence in NRXP's ability to execute on a scalable, high-margin strategy targeting one of the most underserved and urgent areas of mental health.
For More Information
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Website: www.nrxpharma.com
Chief Business Officer: Matthew Duffy
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on illi News
- High-Impact Mental Health Platform Approaching a Defining Regulatory Moment: Eclipsing 70,000 Patients on Real World Use of Ketamine: N ASDAQ: NRXP
- CryptaBox Introduces a Hardware Crypto Cold Storage Wallet
- YWWSDC Launches AI-Native Digital Asset Infrastructure, Merging Technical Innovation with US-Standard Compliance
- High-End Exterior House Painting in Boulder, Colorado
- Simpson and Reed Co-Founders Shardé Simpson, Esq. and Ciara Reed, Esq. Launch "Hello Wilma,"
- Naperville Police Arrest Two Teens Charged with Sexually Assaulting a Female
- Report Outlines Key Questions for Individuals Exploring Anxiety Treatment Options in Toronto
- Rande Vick Introduces Radical Value, Challenging How Brands Measure Long-Term Value
- Chicago: Mayor Brandon Johnson Statement On Trump Threatening To Halt Federal Funding For Sanctuary Cities
- Chicago: Mayor Brandon Johnson Statement On Trump Revoking Protected Status For Somali Immigrants
- Mayor Brandon Johnson And The Chicago Department Of Business Affairs And Consumer Protection Reaffirm Support For Small Business Community At The Start Of The New Year
- Lisa Mauretti Launches Peace of Mind Travel Coaching to Guide Fearful Travelers to Discover the World with Confidence
- New Year, New Home: Begin 2026 at Heritage at South Brunswick
- Food Journal Magazine Releases Its 'Best Food In Los Angeles Dining' Editorial Section
- Enders Capital: 25% Gains with Just -0.80% Maximum Monthly Drawdown in Volatile Debut Year 2025
- Chicago: Mayor's Office Of Reentry Releases Inaugural 2025 End Of Year Report
- CES Spotlight Highlights Need for Strategic Review as Throughput Demands Evolve
- ASR Media, Social T Marketing & PR Announce Merger
- $780,000 Project for New Middle East Police Service with Deposit Received and Preliminary Design Work Underway for Lamperd: Stock Symbol: LLLI
- The 3rd Annual Newark Summit for Real Estate, Economic Development & Placemaking Returns February 9th