Trending...
- Phinge CEO Ranked #1 Globally by Crunchbase for the Last Week, Will Be in Las Vegas Jan. 4-9, the Week of CES to Discuss Netverse & IPO Coming in 2026
- Holiday Pond Lighting Ideas for Chicago-Area Water Features
- Tokenized Real-World Assets: Iguabit Brings Institutional Investment Opportunities to Brazil
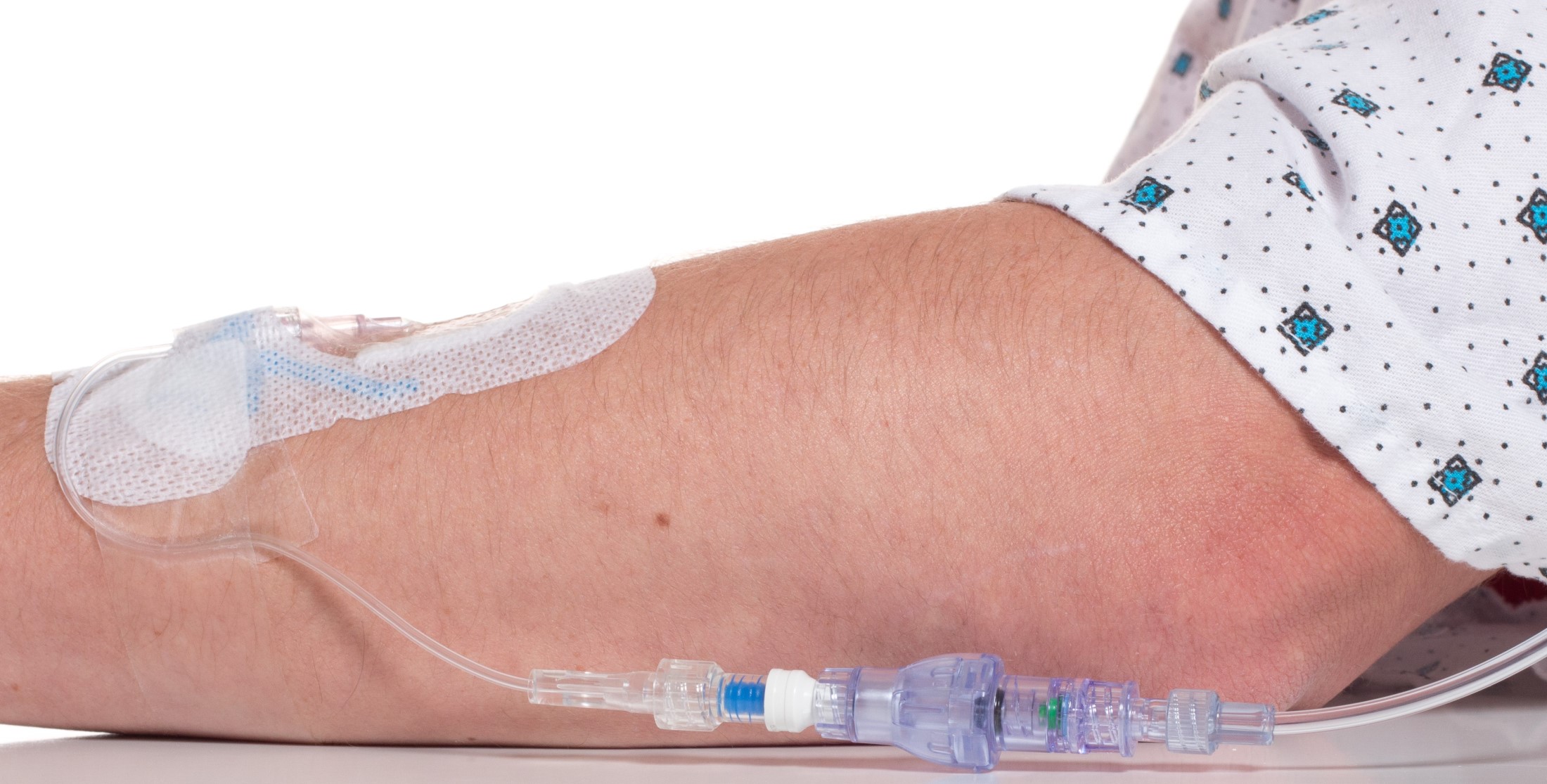
Lineus Medical Creates New Class of Medical Device Intended to Reduce Complications in Peripheral IV Lines
FAYETTEVILLE, Ark. - illiNews -- The US Food and Drug Administration has cleared for sale Lineus Medical's first medical device, SafeBreak Vascular, that reduces mechanical complications requiring IV restarts in peripheral IV lines when excessive tension (greater than 4 lbs. of force) is placed on the IV line. Tugs or pulls on IV lines are a common occurrence and part of every patient's time in the hospital. The current method for securing IV catheters includes the use of adhesive dressings and securement devices. All current methods focus on securing the line to the patient's skin and these solutions fail when the IV tubing is subjected to large pull forces. SafeBreak Vascular works differently than current methods and is part of a new class of devices called Force-Activated Separation Devices. Force-Activated Separation Devices separate to remove the damaging force from the IV line and prevent the loss of blood and medication.
SafeBreak Vascular is the first ever Force-Activated Separation Device cleared to be sold in the United States through the FDA's De Novo review process. Upon separation, a valve in each end of SafeBreak closes. On the patient side, the IV is left intact and the valve closes and prevents the patient from bleeding. On the IV pump side, the valve closes and stops the pump from pumping medicine onto the floor or in the patient's bed. When the fluid flow from the IV pump is stopped, it causes the pump to alarm, letting the medical team know they need to address the situation. The separated SafeBreak Vascular can be discarded and a new one installed in the line in only a few minutes. The patient's medical treatment can resume by restarting the IV infusion without the need for a new catheter or additional needle sticks.
More on illi News
The clinical literature shows that over 235 million peripheral IV lines are placed each year in the United States and that these IV lines fail before their intended end of use a staggering 46% of the time on average. The cost of an IV restart is approximately $50, and IV restarts are estimated to cost the US healthcare system over $5 billion annually.
Lineus Medical was founded in 2015 to develop and bring SafeBreak Vascular to market. "Achieving FDA De Novo Designation as a Class II device is no doubt the biggest milestone to date for our company. We're ecstatic about reaching this inflection point and eager to start executing on our product launch plan. It took an incredible amount of perseverance, a lot of investment, and a talented team to overcome the challenges we faced along the way.", said Lineus Medical's founder and Chief Technology Officer, Spencer Jones.
Vance Clement, CEO of Lineus Medical, stated, "We want to thank our investors for their continued support and especially through the Covid-19 pandemic. A randomized clinical trial that was completed in 2021 showed the device worked as advertised, eliminating spills, and saving nurses a great deal of time. The study most importantly showed a 44% reduction of peripheral IV complications requiring an IV restart in the SafeBreak Vascular group when compared to the control group. Now we are excited to get the product into the hands of clinicians everywhere so that we can start to positively impact a lot more patient's lives."
More on illi News
SafeBreak Vascular is distributed on behalf of Lineus Medical by MediCore of Nashville, TN along with multiple other independent sub-distributors across the entire United States. MediCore distributes other vascular access products to over 1100 US hospitals.
Spencer Jones said, "As a nurse myself, I know that during most shifts nurses are just worried about keeping their heads above water. There's too much to do and never enough time. Empowering nurses is core to our company's mission, and I'm thrilled that we're finally able to offer nurses a device that saves them time, helps reduce stressful IV restarts, and helps them start saving lines. Our team is looking forward to an exciting launch!"
ABOUT LINEUS MEDICAL
Lineus Medical is the developer of a unique, patented, break-away technology that is designed to work with all types of medical tubing. Complications with feeding tubes, IV lines, urinary catheters and any other type of medical tubing may be reduced as force-activated separation devices separate to protect the patient. More information about Lineus Medical can be found on the company website: www.lineusmed.com or you can follow the company on Twitter or LinkedIn.
MKG-0095 06/21 Rev 00
SafeBreak Vascular is the first ever Force-Activated Separation Device cleared to be sold in the United States through the FDA's De Novo review process. Upon separation, a valve in each end of SafeBreak closes. On the patient side, the IV is left intact and the valve closes and prevents the patient from bleeding. On the IV pump side, the valve closes and stops the pump from pumping medicine onto the floor or in the patient's bed. When the fluid flow from the IV pump is stopped, it causes the pump to alarm, letting the medical team know they need to address the situation. The separated SafeBreak Vascular can be discarded and a new one installed in the line in only a few minutes. The patient's medical treatment can resume by restarting the IV infusion without the need for a new catheter or additional needle sticks.
More on illi News
- Chicago: Mayor Brandon Johnson, Office Of Reentry Host Holiday Toy Drive For Returning Residents
- CU Aerospace DUPLEX Satellite Deployed from ISS
- Pinealage: the app that turns strangers into meditation companions — in crowdfunding phase
- "Micro-Studio": Why San Diegans are Swapping Crowded Gyms for Private, One-on-One Training at Sweat Society
- Beycome Closes $2.5M Seed Round Led by InsurTech Fund
The clinical literature shows that over 235 million peripheral IV lines are placed each year in the United States and that these IV lines fail before their intended end of use a staggering 46% of the time on average. The cost of an IV restart is approximately $50, and IV restarts are estimated to cost the US healthcare system over $5 billion annually.
Lineus Medical was founded in 2015 to develop and bring SafeBreak Vascular to market. "Achieving FDA De Novo Designation as a Class II device is no doubt the biggest milestone to date for our company. We're ecstatic about reaching this inflection point and eager to start executing on our product launch plan. It took an incredible amount of perseverance, a lot of investment, and a talented team to overcome the challenges we faced along the way.", said Lineus Medical's founder and Chief Technology Officer, Spencer Jones.
Vance Clement, CEO of Lineus Medical, stated, "We want to thank our investors for their continued support and especially through the Covid-19 pandemic. A randomized clinical trial that was completed in 2021 showed the device worked as advertised, eliminating spills, and saving nurses a great deal of time. The study most importantly showed a 44% reduction of peripheral IV complications requiring an IV restart in the SafeBreak Vascular group when compared to the control group. Now we are excited to get the product into the hands of clinicians everywhere so that we can start to positively impact a lot more patient's lives."
More on illi News
- Tru by Hilton Columbia South Opens to Guests
- Christy Sports donates $56K in new gear to SOS Outreach to help kids hit the slopes
- "BigPirate" Sets Sail: A New Narrative-Driven Social Casino Adventure
- Digi 995 Unveils New Official Website and Shop, Expanding the Digiverse
- Phinge CEO Ranked #1 Globally by Crunchbase for the Last Week, Will Be in Las Vegas Jan. 4-9, the Week of CES to Discuss Netverse & IPO Coming in 2026
SafeBreak Vascular is distributed on behalf of Lineus Medical by MediCore of Nashville, TN along with multiple other independent sub-distributors across the entire United States. MediCore distributes other vascular access products to over 1100 US hospitals.
Spencer Jones said, "As a nurse myself, I know that during most shifts nurses are just worried about keeping their heads above water. There's too much to do and never enough time. Empowering nurses is core to our company's mission, and I'm thrilled that we're finally able to offer nurses a device that saves them time, helps reduce stressful IV restarts, and helps them start saving lines. Our team is looking forward to an exciting launch!"
ABOUT LINEUS MEDICAL
Lineus Medical is the developer of a unique, patented, break-away technology that is designed to work with all types of medical tubing. Complications with feeding tubes, IV lines, urinary catheters and any other type of medical tubing may be reduced as force-activated separation devices separate to protect the patient. More information about Lineus Medical can be found on the company website: www.lineusmed.com or you can follow the company on Twitter or LinkedIn.
MKG-0095 06/21 Rev 00
Source: Lineus Medical
Filed Under: Health, Technology
0 Comments
Latest on illi News
- NAFMNP Awarded USDA Cooperative Agreement to Continue MarketLink Program Under FFAB
- Costa Oil - 10 Minute Oil Change Surpasses 70 Locations with Construction of San Antonio, TX Stores — Eyes Growth Via Acquisition or Being Acquired
- LaTerra and Respark Under Contract with AIMCO to Acquire a $455M, 7-Property Chicago Multifamily Portfolio
- Record Revenue, Tax Tailwinds, and AI-Driven Scale: Why Off The Hook YS Inc. Is Emerging as a Standout in the $57 Billion U.S. Marine Market
- VSee Health (N A S D A Q: VSEE) Secures $6.0M At-Market Investment, Accelerates Expansion as Revenues Surge
- Children Rising Appoints Marshelle A. Wilburn as New Executive Director
- STLE Launches Travel Grants Program to Support Tribology and Lubrication Engineering Professionals
- Digi 995: Void Run Pushes the Digiverse Into Its Darkest, Fastest Chapter Yet
- Fairmint CEO Joris Delanoue Elected General Director of the Canton Foundation
- Sleep Basil Mattress Co.'s Debuts New Home Page Showcasing Performance Sleep Solutions for Active Denver Lifestyles
- Bent Danholm Joins The American Dream TV as Central Florida Host
- Tickeron Unveils AI-Powered Trading Insights Aligned with Key Monthly Consumer Data
- 360Massage Launches Custom-Built Massage Chairs Designed to Match Modern Homes
- The Nature of Miracles Celebrates 20th Anniversary Third Edition Published by DreamMakers Enterprises LLC
- City of Naperville Launches New and Improved eBill Portal
- Artificial Intelligence Leader Releases Children's Book on Veterans Day
- Felicia Allen Hits #1 Posthumously with "Christmas Means Worship"
- CCHR Documentary Probes Growing Evidence Linking Psychiatric Drugs to Violence
- Holiday Pond Lighting Ideas for Chicago-Area Water Features
- Tokenized Real-World Assets: Iguabit Brings Institutional Investment Opportunities to Brazil