Trending...
- Chicago: Mayor Johnson Announces $411M Investment into Community Wealth-Building
- Fourteen-Year-Old Naperville Juvenile Charged with Possession of Loaded Semi-automatic Handgun
- Five Aster Awards! Fusion Marketing Group Brings Home Big Wins in 2025!
The research study of this unique jellyfish collagen-based supplement was published in the peer-reviewed medical source, the Journal of Clinical Research and Reports.
TEMECULA, Calif. - illiNews -- Jellyfish Publication-Certified Nutraceuticals, Inc.
Gerald M. Haase, M.D.
Clinical Professor of Surgery, University of Colorado School of Medicine, Aurora, CO.
Neil E. Wolkodoff, PhD
Medical Program Director, Colorado Center for Health and Sports Science, Denver, CO.
May 16, 2025
Certified Nutraceuticals, Inc., a research and product development enterprise in Pauma Valley, California, U.S.A., announces the publication of an exciting new human clinical trial of its proprietary KollaJell™ collagen peptide formulation.
The research study of this unique jellyfish collagen-based supplement was published in the peer-reviewed medical source, the Journal of Clinical Research and Reports. The strength of this prospective, randomized trial is the distinctive utilization of a panel of brain function metrics from two robust, validated instruments, including a computerized neurocognitive test battery and an electroencephalographic (EEG) profile of voltage activation and wave phase pattern. Instead of evaluating only one or two parameters, this investigation assessed seven domains of cognitive function in 23 subjects who consumed KollaJell™ for a period of eight weeks.
More on illi News
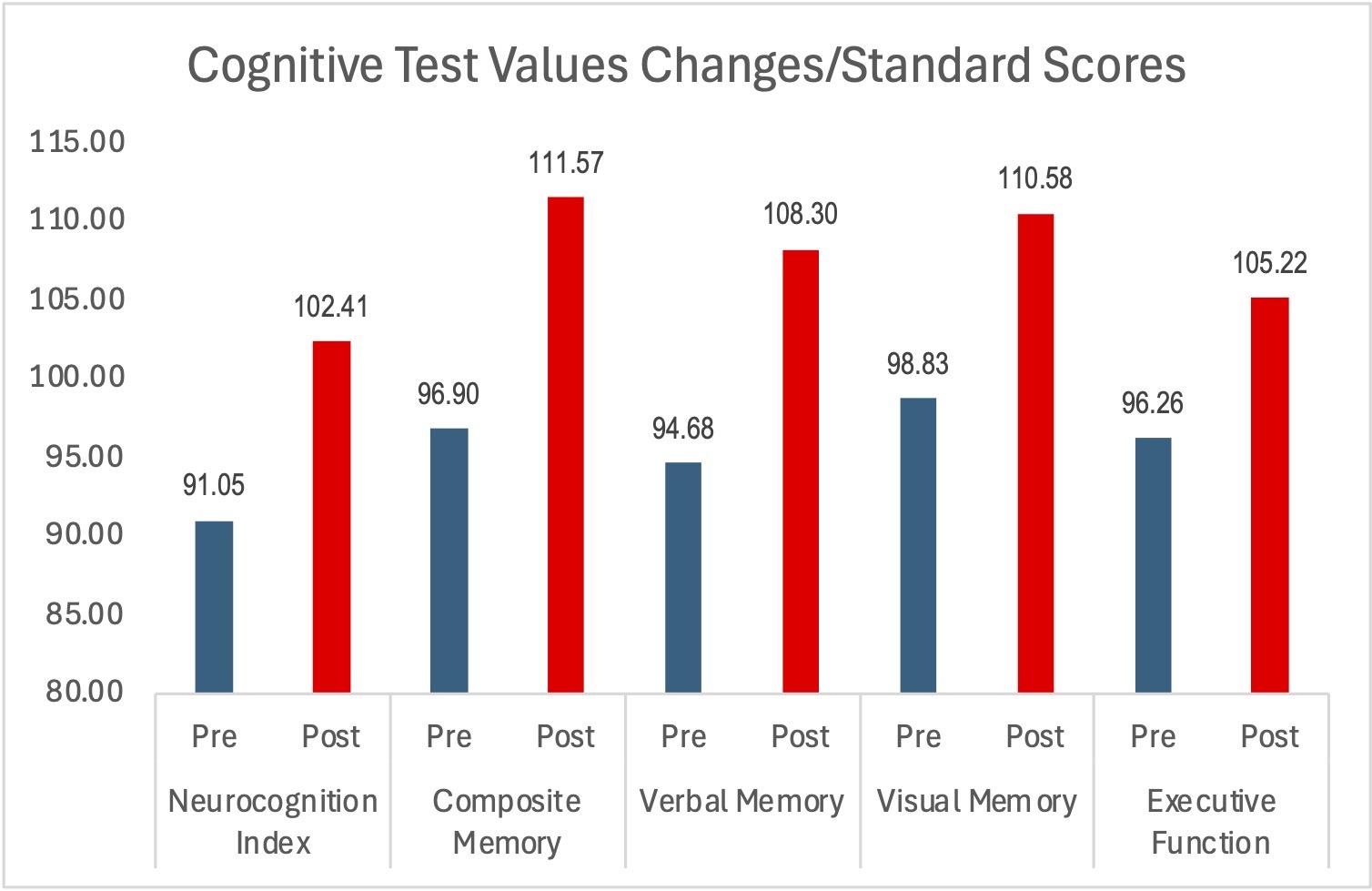
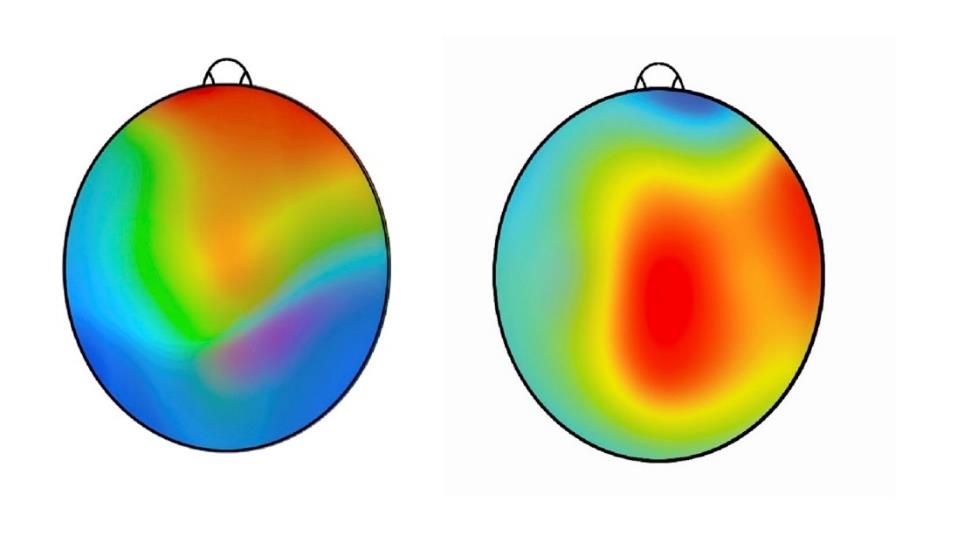
The outcomes were clinically relevant, and all were statistically analyzed and achieved statistical significance. Standard scores matched for age and gender improved in all metrics regardless of the functional status of where the subject started. The parameters included compositive memory, neurocognitive index, visual memory, executive function and verbal memory. Under EEG monitoring conditions, the mean total reaction time and the trail-making composite test times also decreased dramatically. Topographic brain images showed voltage activation in the brain regions associated with sensory input and processing function. There were no adverse effects of consuming the supplement in any subject at any time during the study.
The importance of this pilot trial is the demonstration of a particularly extensive spectrum of beneficial results from the proprietary formulation compared to competing products that, even if helpful, provide only limited effects. In addition, the use of a marine-based natural collagen was superior to the usual animal-based products and the patented extraction process provided a greater platform of collagen types and a uniquely effective amino acid composition. This human clinical trial reinforces the concept that Certified Nutraceuticals KollaJell™ is a safe and beneficial collagen supplement that supports cognitive domains and memory and is applicable to a wide audience of potential consumers, regardless of their initial brain function status.
More on illi News
The actual medical study is available as a free open-access publication at https://www.auctoresonline.org/article/effects-of-a-jellyfish-collagen-based-amino-acid-supplement-on-cognitive-function-and-memory-a-pilot-investigation
For further information about the product or study, please contact:
Sara Alkayali at (951) 600-3899 or email: salkayali@certifiednutra.com
Gerald M. Haase, M.D.
Clinical Professor of Surgery, University of Colorado School of Medicine, Aurora, CO.
Neil E. Wolkodoff, PhD
Medical Program Director, Colorado Center for Health and Sports Science, Denver, CO.
May 16, 2025
Certified Nutraceuticals, Inc., a research and product development enterprise in Pauma Valley, California, U.S.A., announces the publication of an exciting new human clinical trial of its proprietary KollaJell™ collagen peptide formulation.
The research study of this unique jellyfish collagen-based supplement was published in the peer-reviewed medical source, the Journal of Clinical Research and Reports. The strength of this prospective, randomized trial is the distinctive utilization of a panel of brain function metrics from two robust, validated instruments, including a computerized neurocognitive test battery and an electroencephalographic (EEG) profile of voltage activation and wave phase pattern. Instead of evaluating only one or two parameters, this investigation assessed seven domains of cognitive function in 23 subjects who consumed KollaJell™ for a period of eight weeks.
More on illi News
- Pikmykid Partners with Vivi to Enhance School Emergency Communication and Safety
- AI Meets Cybersecurity: IQSTEL and Cycurion Take Aim at $500 Billion Market Opportunity
- N A S D A Q Compliance Achieved Following Active Trading and Financing, UAE Acquisition & Major Brand Events: Lottery.com Inc., (N A S D A Q: LTRY)
- New Frontier Aerospace Successfully Tests Its Revolutionary Mjölnir Rocket Engine
- Profiting from Elder Harm: The Push to End Psychiatric Drugging in Nursing Homes
The outcomes were clinically relevant, and all were statistically analyzed and achieved statistical significance. Standard scores matched for age and gender improved in all metrics regardless of the functional status of where the subject started. The parameters included compositive memory, neurocognitive index, visual memory, executive function and verbal memory. Under EEG monitoring conditions, the mean total reaction time and the trail-making composite test times also decreased dramatically. Topographic brain images showed voltage activation in the brain regions associated with sensory input and processing function. There were no adverse effects of consuming the supplement in any subject at any time during the study.
The importance of this pilot trial is the demonstration of a particularly extensive spectrum of beneficial results from the proprietary formulation compared to competing products that, even if helpful, provide only limited effects. In addition, the use of a marine-based natural collagen was superior to the usual animal-based products and the patented extraction process provided a greater platform of collagen types and a uniquely effective amino acid composition. This human clinical trial reinforces the concept that Certified Nutraceuticals KollaJell™ is a safe and beneficial collagen supplement that supports cognitive domains and memory and is applicable to a wide audience of potential consumers, regardless of their initial brain function status.
More on illi News
- Coalfire Sets the Bar for Responsible AI, Unveiling Industry's First ISO 42001 Certification and Comprehensive Model Testing Program
- LET Mining launches zero-cost cloud mining, daily rewards + referral double benefits
- Oral Semaglutide Significantly Improves Cardiovascular Outcomes in Individuals with Type 2 Diabetes
- Once-Weekly Insulin Efsitora Achieves Comparable A1C Reduction to Daily Insulin Therapy
- CagriSema Demonstrates Significant Weight Loss in Adults with Obesity
The actual medical study is available as a free open-access publication at https://www.auctoresonline.org/article/effects-of-a-jellyfish-collagen-based-amino-acid-supplement-on-cognitive-function-and-memory-a-pilot-investigation
For further information about the product or study, please contact:
Sara Alkayali at (951) 600-3899 or email: salkayali@certifiednutra.com
Source: Certified Nutraceuticals, Inc.
0 Comments
Latest on illi News
- Durex Products Wire Cloth Screen Media: Engineered for Maximum Performance and Durability
- OPRAH.COM Featured Award-Winning Novel AS FAR AS YOU GO BEFORE YOU HAVE TO COME BACK now Available as Audiobook
- How Kallie Boxell Helps Texas Companies Solve the Talent Equation
- Naperville: Structure Fire in the 1500 Block of West Jefferson Avenue
- KeysCaribbean Vacation Home Rentals Offers Last-Minute Booking Discount of 15 Percent
- purelyIV Blog Named One of the Top 45 IV Therapy Blogs by Feedspot
- purelyIV Launches Mobile Iron Infusion Therapy for Patients with Iron Deficiency Anemia
- Zhang Financial Sponsors Distinguished Juror Li-Wei Qin and Historic Instrument Selection for Stulberg's 50th Anniversary
- Smile Makers Dental Care Introduces FP1: East Bay's First Robotic-Assisted Full-Arch Implant Solution for Natural, Fixed Smiles
- DCAS College opens new Representative Office in Malaysian Capital Kuala Lumpur
- GMO Miner: Creating a simple, efficient and reliable new cloud mining experience
- DOT Miners launches a new cloud mining platform: low threshold, high transparency, and helps promote the inclusion of global digital assets
- Fray Fitness and Truemed Partner to Enable HSA/FSA-Funded Fitness Equipment Purchases
- Crazy Discount Codes App Transforms Mobile Shopping With Real-Time Deals
- KPC Marketing Services Launches to Revolutionize Legal Marketing for Small and Mid-Sized Law Firms
- Sploot Vets and DeepScan Launch Exclusive Regional U.S. Partnership to Bring Breakthrough Pet DNA Test to Market
- Lifeway Foods Toasts to 40 Years of Kefir with a Celebratory Drone Show in Chicago
- Naperville: Court Grants State's Motion to Deny Pre-Trial Release for Elgin Man Accused of Attempt Murder
- Various Measures Introduced to and Approved by The Chicago City Council
- As Sober.Buzz Community Explodes It's Growth Globally it is Announcing "Spreading the Good BUZZ" Podcast Hosted by Josh Case Debuting July 7th