Trending...
- Price Improvement on Luxurious Lāna'i Townhome with Stunning Ocean Views
- Trump's Executive Order Rescheduling Cannabis: Accelerating M&A in a Multibillion-Dollar Industry
- Chicago: Mayor Brandon Johnson Signs "Fair Recovery" Executive Order, Prohibiting Sale of Medical Debt, Establishing Responsible Debt Collection Standards
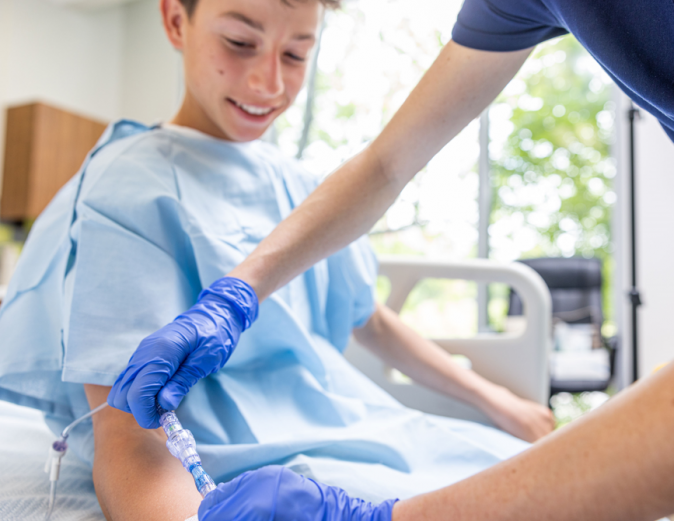
FAYETTEVILLE, Ark. - illiNews -- Lineus Medical, a medical device company focused on reducing IV complications, announced that the company's flagship product, SafeBreak Vascular, has received clearance from the U.S. Food and Drug Administration (FDA) for use in pediatric patients. SafeBreak Vascular has been shown in adult clinical studies to reduce IV complications with its innovative breakaway technology1.
SafeBreak Vascular is the first breakaway device cleared by the FDA and proven to reduce IV complications in adults by 44%1. It fits between the IV tubing from the IV pump and the patient's IV access point at the arm. When there is a damaging force on the line, SafeBreak separates to protect the patient's IV. This innovative feature helps prevent accidental dislodgement of the catheter, as well as other IV complications caused by damaging forces on the IV line1. This is especially important for pediatric patients who may be more active, less inclined to follow instructions, and less aware of their surroundings.
More on illi News
"We are incredibly proud to receive clearance from the FDA for the use of SafeBreak Vascular with pediatric patients," said Vance Clement, CEO of Lineus Medical. "SafeBreak provides a simple, effective solution for preventing IV complications, which reduces IV restarts and additional needlesticks for patients. Now, it doesn't matter if the patient is 1 or 100 years old, SafeBreak is cleared for use on all peripheral IVs. Our goal is to have a SafeBreak on every IV line, everywhere in the world, and this additional clearance lets us help one of the most vulnerable populations that needs it the most, our kids."
ABOUT LINEUS MEDICAL
Lineus Medical is the developer of a unique, patented, break-away technology that is designed to work with all types of medical tubing. Complications with feeding tubes, IV lines, urinary catheters and any other type of medical tubing may be reduced as breakaway devices separate to protect the patient. More information about Lineus Medical can be found on the company website: www.lineusmed.com or you can follow the company on Twitter or LinkedIn.
1 Dipper Study Data on File
MKG-0194 REV 00
SafeBreak Vascular is the first breakaway device cleared by the FDA and proven to reduce IV complications in adults by 44%1. It fits between the IV tubing from the IV pump and the patient's IV access point at the arm. When there is a damaging force on the line, SafeBreak separates to protect the patient's IV. This innovative feature helps prevent accidental dislodgement of the catheter, as well as other IV complications caused by damaging forces on the IV line1. This is especially important for pediatric patients who may be more active, less inclined to follow instructions, and less aware of their surroundings.
More on illi News
- Inkdnylon Launches Ask Inkdnylon Decision Platform
- Phinge Founder & CEO Robert DeMaio Ranked #1 Globally on Crunchbase, Continues to Convert Previous Debt Owed to Him by Phinge into Convertible Notes
- A Chicago Woman Dies In A Shooting-related Car Crash
- Donna Cardellino Manager/Facilitator Signs Justin Jeansonne Country Singer-Songwriter To Exclusive Management Deal For Global Music Expansion
- Golden Paper Launches a New Chapter in Its Americas Strategy- EXPOPRINT Latin America 2026 in Brazil
"We are incredibly proud to receive clearance from the FDA for the use of SafeBreak Vascular with pediatric patients," said Vance Clement, CEO of Lineus Medical. "SafeBreak provides a simple, effective solution for preventing IV complications, which reduces IV restarts and additional needlesticks for patients. Now, it doesn't matter if the patient is 1 or 100 years old, SafeBreak is cleared for use on all peripheral IVs. Our goal is to have a SafeBreak on every IV line, everywhere in the world, and this additional clearance lets us help one of the most vulnerable populations that needs it the most, our kids."
ABOUT LINEUS MEDICAL
Lineus Medical is the developer of a unique, patented, break-away technology that is designed to work with all types of medical tubing. Complications with feeding tubes, IV lines, urinary catheters and any other type of medical tubing may be reduced as breakaway devices separate to protect the patient. More information about Lineus Medical can be found on the company website: www.lineusmed.com or you can follow the company on Twitter or LinkedIn.
1 Dipper Study Data on File
MKG-0194 REV 00
Source: Lineus Medical
Filed Under: Health
0 Comments
Latest on illi News
- A Well-Fed World, Youth Climate Save and PAN International Launch PHRESH: A Global Directory of Plant-Based Hunger Relief Organizations
- Chicago: Mayor Brandon Johnson Signs Executive Order Capping Police Overtime Spending, Creates New Oversight Framework
- Chicago: Mayor Brandon Johnson Signs "Fair Recovery" Executive Order, Prohibiting Sale of Medical Debt, Establishing Responsible Debt Collection Standards
- Guests Can Save 25 Percent Off Last Minute Bookings at KeysCaribbean's Village at Hawks Cay Villas
- Trump's Executive Order Rescheduling Cannabis: Accelerating M&A in a Multibillion-Dollar Industry
- Genuine Hospitality, LLC Selected to Operate Hilton Garden Inn Birmingham SE / Liberty Park
- Documentary "Prescription for Violence: Psychiatry's Deadly Side Effects" Premieres, Exposes Link Between Psychiatric Drugs and Acts of Mass Violence
- Price Improvement on Luxurious Lāna'i Townhome with Stunning Ocean Views
- Nextvisit Co-Founder Ryan Yannelli Identifies Six Critical Factors for Behavioral Health Providers Evaluating AI Scribes in 2026
- Digi 995 Expands Its Puzzle Lineup With the Release of Digi 995: Word Maze on Mobile Platforms
- CredHub and Real Property Management Join Forces to Empower Franchise Owners with Rental Payment Credit Reporting Solutions
- Leimert Park Announces Weeklong Kwanzaa Festival & Kwanzaa Parade Celebrating Black History, Culture, and Community
- Renowned Alternative Medicine Specialist Dr. Sebi and His African Bio Mineral Balance Therapy Are the Focus of New Book
- Psychiatric Drug Damage Ignored for Decades; CCHR Demands Federal Action
- Phil Marley Releases Two Music Projects While Seeking Kidney Donor
- Why Millions Are Losing Sexual Sensation, And Why It's Not Age, Hormones, or Desire
- Justin Jeansonne An Emerging Country Singer-Songwriter Music Fans Have Been Waiting For…a True Maverick
- Russellville Huntington Learning Center Expands Access to Literacy Support; Approved Provider Under Arkansas Department of Education
- UK Financial Ltd Launches U.S. Operations Following Delaware Approval
- Author Charlene Wexler Earns International Impact Book Award for We Won't Go Back