Trending...
- Free and Low-Cost Dental Care Now Available in London Through the Canadian Dental Care Plan (CDCP)
- Garden State Gay Socials Turns One: 1st Birthday Celebration for Gay Men Who Want Real Connection
- London Gala Emphasizes Trade, FDI and Ongoing Cooperation
Devices not cleared by the FDA are being sold in the USA! Buying non-fda cleared device can cause injury. Spa Sciences launches LEXI after receiving FDA clearance for effective treatment. IPL devices are considered medical devices and have rigorous requirements. Spa Sciences launches LEXI IPL device for permanent hair removal after FDA clearance bringing customers a lifetime of hair removal in a single device. Get safe and affordable permanent hair removal in the comfort of your home with LEXI.
PORT ST. LUCIE, Fla. - illiNews -- Spa Sciences, the #1 facial skin device brand in the United States, announces it has been granted FDA clearance for its newest IPL device LEXI for a permanent home-use hair removal solution.
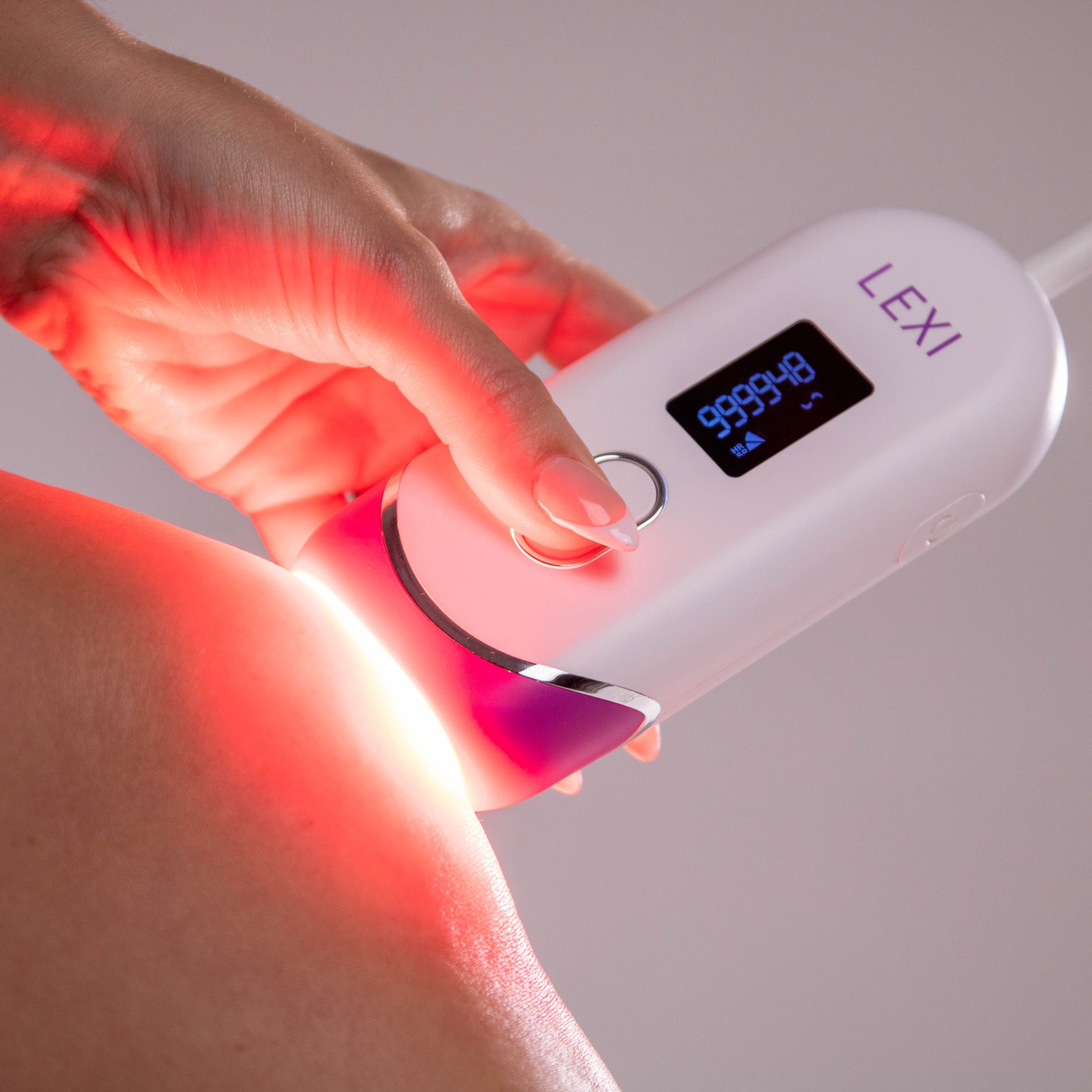
IPL stands for intense pulsed light. It works similarly to laser hair removal, only it's way easier and safer to do at home. LEXI is a handheld device that targets unwanted hairs at the root and destroys them with light pulses without burning or damaging your skin. It stops hair from growing back for long-term hair reduction from the comfort of your home.
More on illi News
An IPL is considered a medical device which requires FDA authorization before it can be sold legally in the U.S. "There are a number of IPL devices to choose from on Amazon and elsewhere the vast majority of which are not legally cleared by the FDA, so consumers need to be careful when purchasing an IPL and make sure the product they select from Amazon is FDA approved or cleared as required by law," said co-founder and president Michael Friend. "Our LEXI IPL hair removal device has undergone rigorous trials to ensure that the device is safe, effective and FDA compliant so our customers can purchase LEXI with confidence, knowing treatment will be effective and safe."
About LEXI by Spa Sciences
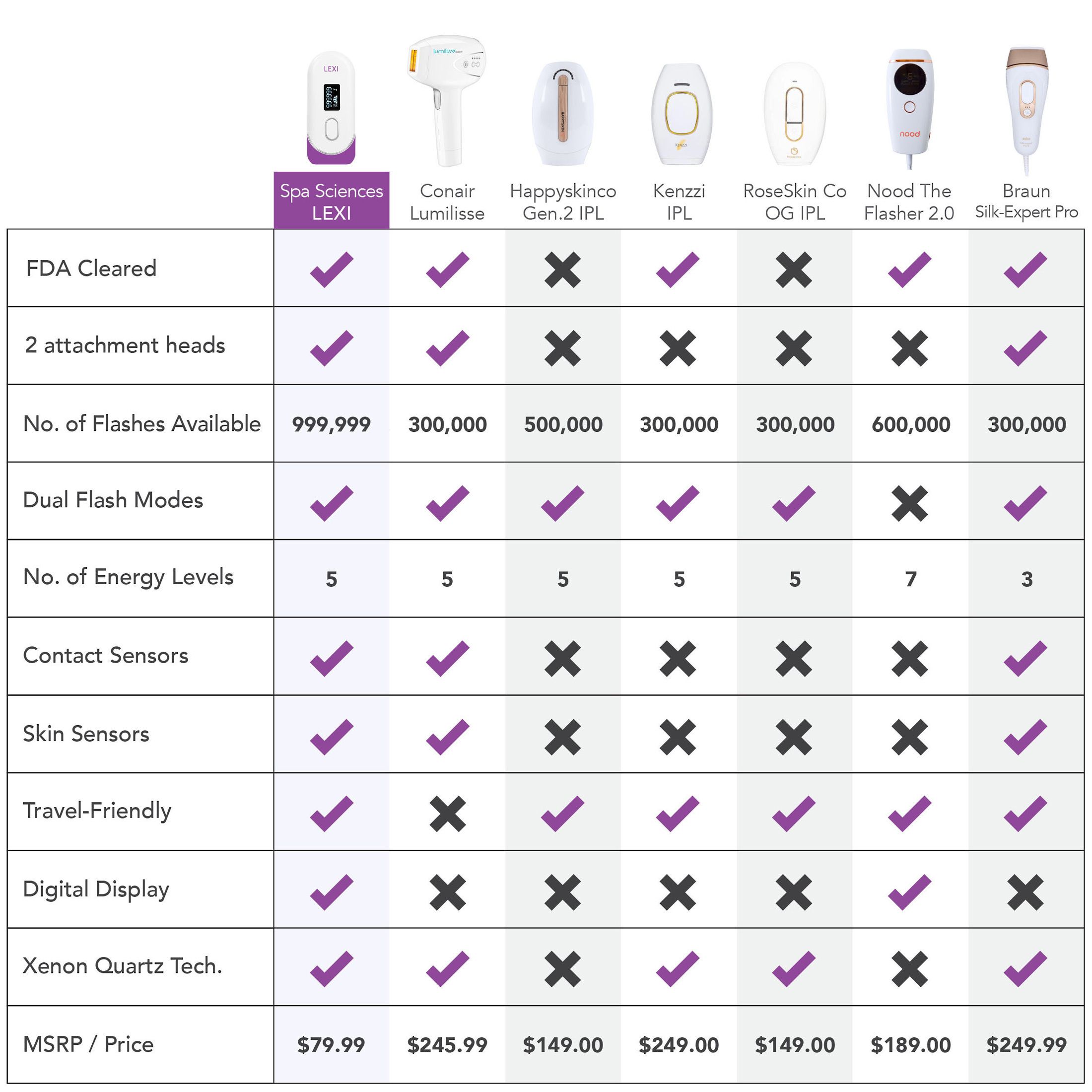
LEXI is a noninvasive Intensive Pulse Light therapy device, FDA cleared for the removal of unwanted hair long-term or permanently. Boasting an impressive 1,000,000 flashes capacity - enough for a lifetime of hair removal - the cutting-edge hair removal technology of LEXI is incredibly effective at visibly reducing or eliminating hair growth and features auto flash, skin sensors, and digital screen for easy and safe hair removal.
More on illi News
https://youtu.be/VbmrMc1VxQE
About Spa Sciences
Spa Sciences is the #1 facial skin device brand in the U.S., according to Nielsen, a global leader in audience measurement, data, and analytics. Spa Sciences is a leading expert in developing and innovating affordable skin technologies that have redefined at-home skin care treatments to bring the spa experience home through advanced beauty & wellness devices.
IPL stands for intense pulsed light. It works similarly to laser hair removal, only it's way easier and safer to do at home. LEXI is a handheld device that targets unwanted hairs at the root and destroys them with light pulses without burning or damaging your skin. It stops hair from growing back for long-term hair reduction from the comfort of your home.
More on illi News
- Mid-West Moving & Storage 2025 Best Places to Work
- HABEMUS PAPAM - We Have a Pope!
- A.J. Rhem & Associates Launches AI Center of Excellence to Drive Ethical and Scalable AI Innovation
- Mad Farmer Tour Brings National Spotlight to Chicago Block Leaders Reclaiming Soil and Community
- Fairmint Introduces First Fully Onchain and Open Cap Table Infrastructure
An IPL is considered a medical device which requires FDA authorization before it can be sold legally in the U.S. "There are a number of IPL devices to choose from on Amazon and elsewhere the vast majority of which are not legally cleared by the FDA, so consumers need to be careful when purchasing an IPL and make sure the product they select from Amazon is FDA approved or cleared as required by law," said co-founder and president Michael Friend. "Our LEXI IPL hair removal device has undergone rigorous trials to ensure that the device is safe, effective and FDA compliant so our customers can purchase LEXI with confidence, knowing treatment will be effective and safe."
About LEXI by Spa Sciences
LEXI is a noninvasive Intensive Pulse Light therapy device, FDA cleared for the removal of unwanted hair long-term or permanently. Boasting an impressive 1,000,000 flashes capacity - enough for a lifetime of hair removal - the cutting-edge hair removal technology of LEXI is incredibly effective at visibly reducing or eliminating hair growth and features auto flash, skin sensors, and digital screen for easy and safe hair removal.
More on illi News
- Vortex Brands Begins Gold Purchases Under New Joint Venture with Dubai-Based Partner
- Chicago: Mayor Brandon Johnson Statement on the Election of Pope Leo XIV
- City of Naperville Fleet Services Division Ranks No. 7 in the NAFA 100 Best Fleets Competition
- This Budget Didn't Break the System—It Exposed the Truth
- Statement From EWTN Chairman & CEO Michael P. Warsaw On The Election of Pope Leo XIV
https://youtu.be/VbmrMc1VxQE
About Spa Sciences
Spa Sciences is the #1 facial skin device brand in the U.S., according to Nielsen, a global leader in audience measurement, data, and analytics. Spa Sciences is a leading expert in developing and innovating affordable skin technologies that have redefined at-home skin care treatments to bring the spa experience home through advanced beauty & wellness devices.
Source: Spa Sciences
0 Comments
Latest on illi News
- Transfer Pricing Expert Andrew O'Brien-Penney Joins The Brattle Group as Principal
- Naperville Fire Department Invites Community Members to Fire Prevention Presentation on May 22
- L2 Aviation Celebrates Grand Opening of New Facility at Cincinnati/Northern Kentucky International Airport (CVG)
- Managing Summer Staffing Surges with Confidence: Why Name Badges Are a Must for Seasonal Success
- DR Instruments Inc Expands Lab Apparel Line with New 100% Cotton Lab Coats for Adults and Youth
- Visa Named Title Sponsor of Ascending Athletes' Business Owners Summits for NFL Entrepreneurs
- The Paris Court of International Arbitration Elects Dr. John J. Maalouf as its New President
- Powerhouse Dynamics to Showcase Groundbreaking OilSmart by Open Kitchen® Solution at National Restaurant Association Show
- $56.7 Million Announced in Q1 2025 with Revenue Growth and Progress Toward NASDAQ Uplisting for AI Marketing Company: IQSTEL, Inc. Stock Symbol: IQSTD
- SAVVY MINING raised $500 million and launched BTC.XRP.DOGE cloud mining, increasing investors' returns by 30%
- New National Nonprofit Launches to Capture Firsthand Accounts of Adoption Stories
- The Tide Project Opens at Biennale Architettura 2025 in Venice Amplifying Youth Voices
- Wall Street analysts say BTC.XRP.DOGE cloud mining company SIX MINING is expected to achieve a 5-fold increase, allowing users to easily mine BTC
- Gen X Takes The Reins: New Book Guides Caregivers Juggling Parents, Kids, And Grandkids With Humor And Heart
- Naperville Welcomes Top Global Leaders Through Americas Competitiveness Exchange Visit
- Fray Fitness Launches Memorial Day Sale and Veteran Organization Giveaway
- ABM for Good™ Launches First Project with Build Change
- Local Nonprofit Files Lawsuit Against Trump Administration's Attack on AmeriCorps
- Pregis Honors Partners Driving Measurable Impact with Annual Pregis Purpose Awards
- ImagineX, in Collaboration with Qualys, Launches New mROC Services to Transform Enterprise Cyber Risk Management