Trending...
- Hiclean Tools Releases HCX2100 Electric Pressure Washer
- Capacity Crunch for Cold Plating? Not Anymore
- Some Music for Donald's Bad Day
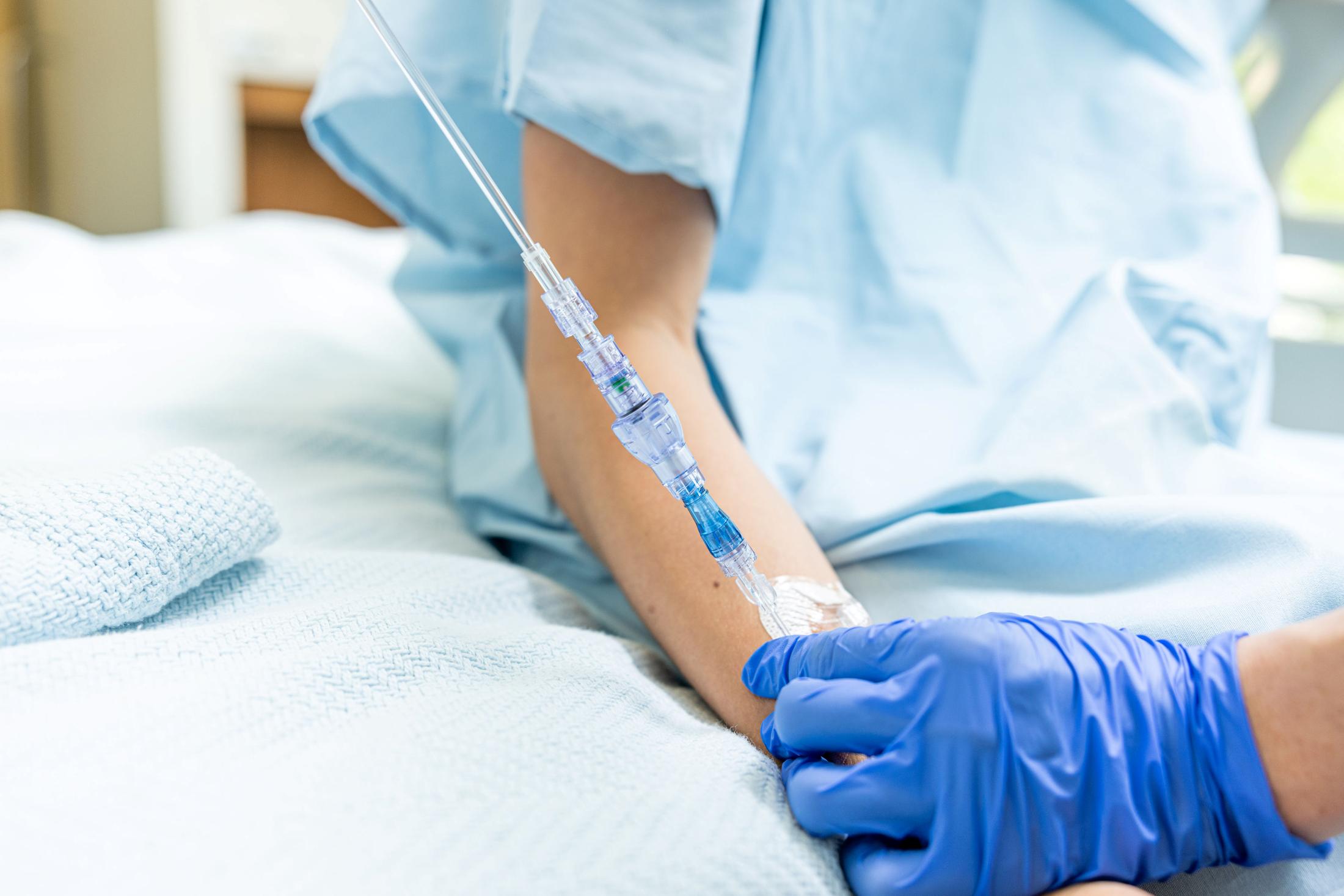
FAYETTEVILLE, Ark. - illiNews -- Lineus Medical has obtained CE Mark certification for its flagship product, SafeBreak® Vascular, the only breakaway device for IV lines clinically proven to reduce multiple IV complications.1 With this certification, SafeBreak Vascular is now cleared for use across the European Union. This approval expands SafeBreak Vascular's clearance from 5 countries to 32, spanning across more than three continents. Lineus Medical is actively partnering with distributors across Europe to expand hospital access to SafeBreak worldwide.
SafeBreak Vascular is a break-away device for IV lines clinically proven to reduce IV complications1. When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patent's infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money1.
More on illi News
"Earning the CE Mark is a major milestone in our company's journey," says Vance Clement, CEO of Lineus Medical. "It reflects our commitment to remove the pains associated with IV lines and make SafeBreak Vascular the standard of care worldwide. Our goal is to be the leading company in this market segment in both science and sales. This clearance affirms that position and makes SafeBreak the first break-away device to have clearance in Asia, Europe, and North America."
Larry Hayes, VP of Sales comments, "With CE Mark approval, we are opening the door to new distribution partnerships across Europe. This certification allows SafeBreak Vascular to reach more hospitals and healthcare systems, accelerating our global commercialization strategy and expanding our global footprint."
"Achieving CE Mark certification is a testament to the quality and reliability of both our product and our manufacturing processes," said Will Armstrong, COO. "It reflects the strength of our team and our commitment to maintaining the highest standards."
About Lineus Medical
Lineus Medical is the developer of SafeBreak Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References:
1. Data on File
SafeBreak Vascular is a break-away device for IV lines clinically proven to reduce IV complications1. When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patent's infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money1.
More on illi News
- i2 Group Acquisitions and Investments in Innovations Deliver 40% Increase in Year-on-Year Bookings
- New Book Release: The Tree That Could Not Change
- BayWa r.e. Solar Trade and WHES Announce Distribution Partnership for the European Market: Delivering Smarter Energy Storage
- Fleet Mining Cloud Mining Platform — Latest Guide: Making Bitcoin Mining Safer and More Convenient
- Leo Choir × Confidence Apparel: From Fashion Week to the Chicago Bulls Arena
"Earning the CE Mark is a major milestone in our company's journey," says Vance Clement, CEO of Lineus Medical. "It reflects our commitment to remove the pains associated with IV lines and make SafeBreak Vascular the standard of care worldwide. Our goal is to be the leading company in this market segment in both science and sales. This clearance affirms that position and makes SafeBreak the first break-away device to have clearance in Asia, Europe, and North America."
Larry Hayes, VP of Sales comments, "With CE Mark approval, we are opening the door to new distribution partnerships across Europe. This certification allows SafeBreak Vascular to reach more hospitals and healthcare systems, accelerating our global commercialization strategy and expanding our global footprint."
"Achieving CE Mark certification is a testament to the quality and reliability of both our product and our manufacturing processes," said Will Armstrong, COO. "It reflects the strength of our team and our commitment to maintaining the highest standards."
About Lineus Medical
Lineus Medical is the developer of SafeBreak Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References:
1. Data on File
Source: Lineus Medical
0 Comments
Latest on illi News
- The OpenSSL Corporation and the OpenSSL Foundation Celebrate the Success of the Inaugural OpenSSL Conference in Prague
- TKL Group's New Factory Commences Production, Pioneering A New Era In Global Heavy Duty Truck Parts
- Regulated Crypto Exchange TZNXG Addresses Core US Market Challenges with Compliance-First Infrastructure
- Unleash Your Inner Seduction at HandPickedSC.com's "The Hot & The Wicked Halloween Soirée"
- GitKraken Launches Insights to Help Engineering Leaders Quantify AI Impact and Improve Developer Experience
- ZapperBox NextGen TV Gateway Receiver Now Testing Support For Secure Whole-Home Content Distribution
- Life as a Dog: P-Wave Press Brings Readers a Heartwarming Memoir of Love, Laughter and Companionship
- NOYA Launches Premium, Design-Forward Training Gear That Belongs at the Center of Your Space
- Research Defense Examines Violence, Illiteracy, Non-Active Fathers, and Low Self-Esteem Among Males
- Investing in Greece: Your Definitive Real-Estate FAQ Guide
- Mayor Brandon Johnson & World Business Chicago Host Chicago Consular Corps Diplomatic Roundtable
- KeysCaribbean Offers 20 Percent Off Seven-Night Stays For Private Home Collection Properties
- Advancing Circular Economy in Automotive ESD Packaging
- Institute for Pet Health Sciences Names Boops Pets 2025 Product of the Year
- Medcor Expands Telemedicine Access with Telecare Anywhere
- Matthew Cossolotto, Author of The Joy of Public Speaking, Appears on "Get Authentic with Marques Ogden" and "Achieving Success with Olivia Atkin"
- CCHR Exposes Conflicted Psychiatrists Behind Teen Antidepressant Surge
- Chicago: O'Hare Marks Busiest Summer Ever, Surpassing Pre-Pandemic Peak For The Season
- WIBO Announces Fall 2025 Entrepreneurship Programs to Empower NYC Founders and Small Business Owners
- Home Equity Theft: How Small Debts Are Costing Americans Their Homes